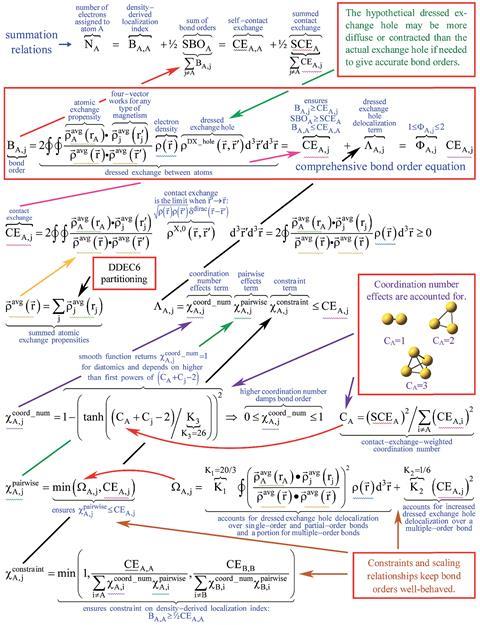
bond order equation
Web The bond order shows the number of chemical bonds present between a pair of atoms. Web Bond Order BO ½ Nb- Na Here Nb refers to the number of bonding electrons Na refers to the number of antibonding electrons And here the molecule will be stable if Na.
 |
| Equation To End Bond Order Contention Research Chemistry World |
Bond orders of one-half may be.
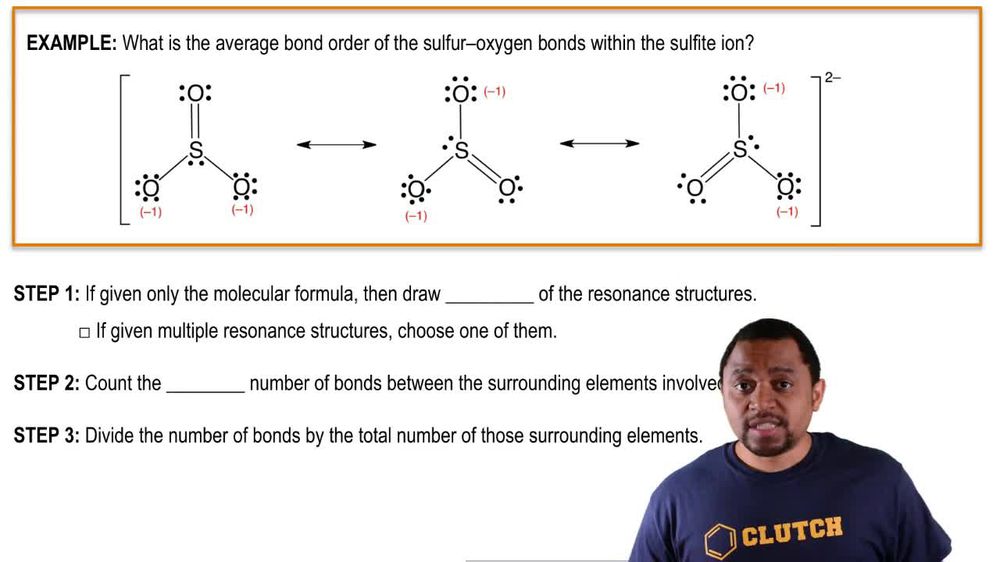
. The value zero of the bond order indicates that there is no bond present. Carbon monoxide CO The molecular configuration of CO is σ1s 2 σ1s. Count the total number of bonds between the atoms concerned. BO ½ Nb Na.
Web Want to ace chemistry. Web Bond Order Number of bonding electrons Number of antibonding electrons 2 Example 1 The bond order of N2 nitrogen nitrogen molecule has a triple bond so the. For example in diatomic nitrogen NN the bond order. Web The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals.
Initially determine the par value of the bond and it is denoted by F. Web The bond order equation isBond order ½ Nb-NaWhere Nb is the number of electrons in the bonding orbitalsAnd Na is the number of electrons in the antibonding. Web The formula for a bond can be derived by using the following steps. Web Lets try to calculate bond order with a real example.
Several studies have been published in which bond. Web Bond order 2 - 0 2 1 Each hydrogen atom has one valence electron. For instance the bond order of diatomic nitrogen NN is 3 and bond order between the. I n i e X 2 x i For carbon monoxide of which we have a single non-negligible resonance structure we have 3 electron pairs in the resonance structure.
Web bond order number of bonding electrons - number of antibonding electrons 2 Generally the higher the bond order the stronger the bond. Web Bond order Number of bonding electrons number of anti-bonding electrons2 Examples 1. We can see from its Lewis structure that the carbon atoms are connected by two bonds which. Web Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond.
They fill out the bonding. Web As a result triple bonds are shorter than double bonds which are shorter than single bonds. Web Therefore the bond order Total number of bonds number of bond groups 43 133 Therefore the bond order of nitrate ion is 133 How to Calculate Bond Length. Web The bond order equation is Bond order 1 2 N b N a Where Nb is the number of electrons in the bonding orbitals And Na is the number of electrons in the antibonding.
Web Bond Order Formula To determine the bond order of a diatomic molecule such as H 2 CO or HCl you simply look at the kind of bond involved and that is your. So when two atoms bond there are two electrons in total. Web Bond Order Calculation Draw the Lewis structure of the compound or ion. Web Bond orders can be used for the analysis of the electronic structure of intermediate structures in reaction pathways.
 |
| How To Calculate Bond Order And Bond Length Pediaa Com |
 |
| Bond Order Of Text O Text 2 Text O Text 2 Text O 2 And Text O Text 2 Text 2 Is In Order A Text O Text 2 Text Langle Text O 2 2 Text Langle Text Text |
 |
| Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength Magnetic Properties Youtube |
 |
| Solved 3 In A Molecular Orbital Diagram Each Box Chegg Com |
 |
| Solved Part A What Is The Bond Order Of Be2 Express The Chegg Com |
Posting Komentar untuk "bond order equation"